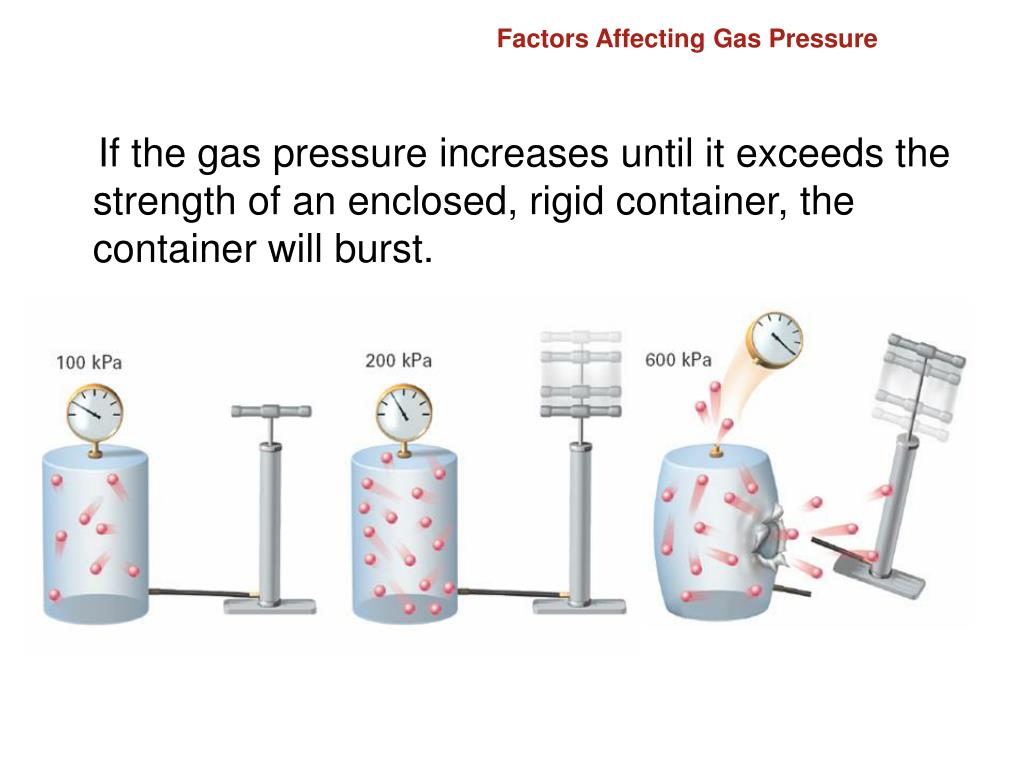
Does Pressure Increase With Temperature . If you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas. According to boyle’s law, we would have to double the pressure to halve the volume. Thus, if the volume of gas is to remain the same, doubling. Learn how pressure and temperature of a gas are related by the kinetic theory and the pressure law. The following figure illustrates the. Find out how to use the equation \\ ( \\frac {p_. The relationships among the volume of a gas and its pressure, temperature, and amount are summarized in figure \(\pageindex{5}\).
from www.slideserve.com
The following figure illustrates the. The relationships among the volume of a gas and its pressure, temperature, and amount are summarized in figure \(\pageindex{5}\). According to boyle’s law, we would have to double the pressure to halve the volume. If you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas. Find out how to use the equation \\ ( \\frac {p_. Learn how pressure and temperature of a gas are related by the kinetic theory and the pressure law. Thus, if the volume of gas is to remain the same, doubling.
PPT Compressibility PowerPoint Presentation, free download ID4200029
Does Pressure Increase With Temperature If you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas. The relationships among the volume of a gas and its pressure, temperature, and amount are summarized in figure \(\pageindex{5}\). The following figure illustrates the. Thus, if the volume of gas is to remain the same, doubling. Learn how pressure and temperature of a gas are related by the kinetic theory and the pressure law. If you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas. According to boyle’s law, we would have to double the pressure to halve the volume. Find out how to use the equation \\ ( \\frac {p_.
From www.savemyexams.co.uk
Gas Law Relationships (1.2.5) IB DP Chemistry SL Revision Notes 2016 Does Pressure Increase With Temperature Learn how pressure and temperature of a gas are related by the kinetic theory and the pressure law. If you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas. According to boyle’s law, we would have to double the pressure to halve the volume. Thus, if the volume of gas. Does Pressure Increase With Temperature.
From www.teachoo.com
Changing Pressure to Change State of Matter Chemistry Teachoo Does Pressure Increase With Temperature The relationships among the volume of a gas and its pressure, temperature, and amount are summarized in figure \(\pageindex{5}\). According to boyle’s law, we would have to double the pressure to halve the volume. The following figure illustrates the. Learn how pressure and temperature of a gas are related by the kinetic theory and the pressure law. Thus, if the. Does Pressure Increase With Temperature.
From www.youtube.com
Partial Pressure Change with Temperature (Interactive) YouTube Does Pressure Increase With Temperature Learn how pressure and temperature of a gas are related by the kinetic theory and the pressure law. If you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas. The following figure illustrates the. Find out how to use the equation \\ ( \\frac {p_. The relationships among the volume. Does Pressure Increase With Temperature.
From www.researchgate.net
Variations of the pressure increase, temperature rise, and film Does Pressure Increase With Temperature Learn how pressure and temperature of a gas are related by the kinetic theory and the pressure law. Thus, if the volume of gas is to remain the same, doubling. The following figure illustrates the. If you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas. According to boyle’s law,. Does Pressure Increase With Temperature.
From imsucc.blogspot.com
Le Chateliers Principle Temperature And Pressure Get Images Does Pressure Increase With Temperature Find out how to use the equation \\ ( \\frac {p_. If you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas. The relationships among the volume of a gas and its pressure, temperature, and amount are summarized in figure \(\pageindex{5}\). Learn how pressure and temperature of a gas are. Does Pressure Increase With Temperature.
From www.slideserve.com
PPT Factors Affecting the Rate of a Chemical Reaction PowerPoint Does Pressure Increase With Temperature Find out how to use the equation \\ ( \\frac {p_. The relationships among the volume of a gas and its pressure, temperature, and amount are summarized in figure \(\pageindex{5}\). Thus, if the volume of gas is to remain the same, doubling. Learn how pressure and temperature of a gas are related by the kinetic theory and the pressure law.. Does Pressure Increase With Temperature.
From www.slideserve.com
PPT Chapter 18 PowerPoint Presentation, free download ID1757364 Does Pressure Increase With Temperature Find out how to use the equation \\ ( \\frac {p_. The following figure illustrates the. According to boyle’s law, we would have to double the pressure to halve the volume. Thus, if the volume of gas is to remain the same, doubling. If you had a way to increase pressure with no volume change, then yes, temperature would increase. Does Pressure Increase With Temperature.
From www.researchgate.net
Variations of the pressure increase, temperature rise, and film Does Pressure Increase With Temperature According to boyle’s law, we would have to double the pressure to halve the volume. Learn how pressure and temperature of a gas are related by the kinetic theory and the pressure law. The following figure illustrates the. The relationships among the volume of a gas and its pressure, temperature, and amount are summarized in figure \(\pageindex{5}\). Find out how. Does Pressure Increase With Temperature.
From www.chemistrystudent.com
Boltzmann Distribution Curves (ALevel) ChemistryStudent Does Pressure Increase With Temperature According to boyle’s law, we would have to double the pressure to halve the volume. Find out how to use the equation \\ ( \\frac {p_. If you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas. The relationships among the volume of a gas and its pressure, temperature, and. Does Pressure Increase With Temperature.
From www.goodscience.com.au
Factors that Affect Rate of Reaction Good Science Does Pressure Increase With Temperature According to boyle’s law, we would have to double the pressure to halve the volume. If you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas. Learn how pressure and temperature of a gas are related by the kinetic theory and the pressure law. Thus, if the volume of gas. Does Pressure Increase With Temperature.
From general.chemistrysteps.com
States of Matter Solid, Liquid, Gas, and Plasma Chemistry Steps Does Pressure Increase With Temperature Find out how to use the equation \\ ( \\frac {p_. According to boyle’s law, we would have to double the pressure to halve the volume. If you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas. Thus, if the volume of gas is to remain the same, doubling. Learn. Does Pressure Increase With Temperature.
From chemwiki.ucdavis.edu
13.4 Effects of Temperature and Pressure on Solubility Chemwiki Does Pressure Increase With Temperature Find out how to use the equation \\ ( \\frac {p_. Learn how pressure and temperature of a gas are related by the kinetic theory and the pressure law. Thus, if the volume of gas is to remain the same, doubling. The relationships among the volume of a gas and its pressure, temperature, and amount are summarized in figure \(\pageindex{5}\).. Does Pressure Increase With Temperature.
From mavink.com
Air Pressure Altitude Chart Does Pressure Increase With Temperature According to boyle’s law, we would have to double the pressure to halve the volume. Learn how pressure and temperature of a gas are related by the kinetic theory and the pressure law. If you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas. Find out how to use the. Does Pressure Increase With Temperature.
From socratic.org
How do mountains affect temperature on both the windward side and the Does Pressure Increase With Temperature The relationships among the volume of a gas and its pressure, temperature, and amount are summarized in figure \(\pageindex{5}\). If you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas. Thus, if the volume of gas is to remain the same, doubling. The following figure illustrates the. Find out how. Does Pressure Increase With Temperature.
From engineerexcel.com
Pressure Temperature Graphs Explained EngineerExcel Does Pressure Increase With Temperature Find out how to use the equation \\ ( \\frac {p_. Thus, if the volume of gas is to remain the same, doubling. If you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas. According to boyle’s law, we would have to double the pressure to halve the volume. The. Does Pressure Increase With Temperature.
From docslib.org
Relationship Between Density, Pressure, and Temperature DocsLib Does Pressure Increase With Temperature The relationships among the volume of a gas and its pressure, temperature, and amount are summarized in figure \(\pageindex{5}\). Learn how pressure and temperature of a gas are related by the kinetic theory and the pressure law. Thus, if the volume of gas is to remain the same, doubling. According to boyle’s law, we would have to double the pressure. Does Pressure Increase With Temperature.
From www.slideserve.com
PPT Unit 4 Phases of Matter (Chapters 1314) PowerPoint Presentation Does Pressure Increase With Temperature Thus, if the volume of gas is to remain the same, doubling. Find out how to use the equation \\ ( \\frac {p_. The relationships among the volume of a gas and its pressure, temperature, and amount are summarized in figure \(\pageindex{5}\). If you had a way to increase pressure with no volume change, then yes, temperature would increase by. Does Pressure Increase With Temperature.
From slidetodoc.com
The relationship between temperature and volume How Volume Does Pressure Increase With Temperature Learn how pressure and temperature of a gas are related by the kinetic theory and the pressure law. The relationships among the volume of a gas and its pressure, temperature, and amount are summarized in figure \(\pageindex{5}\). The following figure illustrates the. Thus, if the volume of gas is to remain the same, doubling. According to boyle’s law, we would. Does Pressure Increase With Temperature.